Biobank Cohorts
BBMRI.at partner biobanks harbour millions of samples and associated data from various disease and health conditions
Millions of samples and associated data from various disease and health conditions are stored at BBMRI.at partner biobanks. Together with the expertise of clinical partners and the know-how and services by the biobanks, they are an extraordinary resource for biomedical R&D.
These samples (human, animal) represent all detected diseases at their natural frequency of occurrence and include also healthy samples.
Major sample types include formalin fixed paraffin embedded (FFPE), and fresh frozen tissues, fluid samples (serum, plasma, whole blood, urine, stool, cerebrospinal, follicular and seminal fluids, etc.), stained tissue sections, and digital (whole slide) images from H&E or Immunohistochemically stained tissue slides.
More about the samples and how to get in contact with BBMRI.at partner biobanks
or write an e-mail to contact@bbmri.at
(contact person: Cornelia Stumptner, BBMRI.at)
Some of many special collections we highlight and describe below
„Spleen diseases in dogs“
An exceptional sample collection at VetBiobank Vienna (University of Veterinary Medicine, Vienna)
„Lighthouse collection: Prostate cohort“
An exceptional sample collection of the Department of Urology at Biobank Innsbruck (Med Uni Innsbruck)
„CoVVac Study: COVID-19 vaccination cohort“
An outstanding sample collection at Biobank Graz (Med Uni Graz)
„Lighthouse collection: COVID-19 Convalescent Cohort“
An exceptional sample collection at Biobank Graz (Med Uni Graz) which is based on a prospective sample cohort for current research on SARS-CoV-2
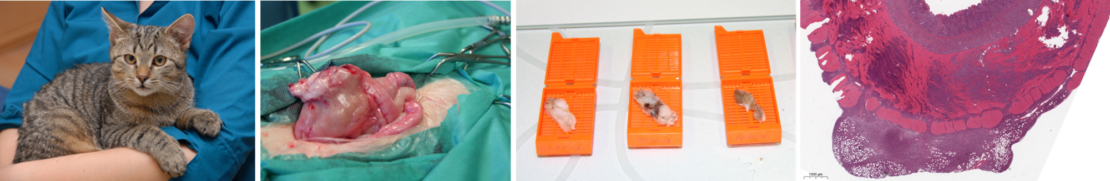
„Lighthouse collection: Feline Lymphoma Cohort“
An exceptional sample collection at VetBiobank Vienna (University of Veterinary Medicine, Vienna)
„Lighthouse collection: Colorectal Cancer Whole Slide Image – Survival Cohort“
An exceptional collection by Diagnostic & Research Institute of Pathology at Biobank Graz (both Med Uni Graz)
„Lighthouse collection: Colorectal Cancer Whole Slide Image – Vascular Invasion Cohort“
An exceptional collection by Diagnostic & Research Institute of Pathology at Biobank Graz (both Med Uni Graz)
„Lighthouse collection: Colorectal Cancer Whole Slide Image – Clinical Annotation Cohort“
An exceptional collection by Diagnostic & Research Institute of Pathology at Biobank Graz (both Med Uni Graz)
„LEAD Cohort (Austrian LEAD Study)“
An outstanding collection at BBMRI.at partner MedUni Wien Biobank
„Lighthouse collection: BioPersMed Cohort“
An outstanding interdisciplinary sample collection at Biobank Graz
„PoCOsteo Osteoporosis Cohort“
An outstanding osteoporosis sample collection at Biobank Graz (Med Uni Graz)
„PERFORM Cohort“
An outstanding sample collection at Biobank Graz (Med Uni Graz)
„INTERFAST Cohort“
An outstanding sample collection at Biobank Graz (Med Uni Graz)
„Paracelsus 10,000 Cohort“
An outstanding population-based collection at BBMRI.at partner PMU / SALK
Spleen diseases in dogs
An exceptional sample collection at VetBiobank Vienna (University of Veterinary Medicine, Vienna) based on a prospective cohort of dogs with splenic masses, established to support current research in veterinary oncology
Splenic masses, which can arise from benign or malignant lesions, are prevalent in dogs and vary dramatically in their clinical behaviors. Benign tumor, such as splenic hematoma and nodular hyperplasia, are non-cancerous masses of clotted blood that can be surgically removed with curative intent. In contrast, malignant lesions, including hemangiosarcomas, lymphomas, and various sarcomas can present as aggressive tumors with a poor prognosis.
Splenic tumors are particularly common in large-breed dogs, with German Shepherds, Golden Retrievers, and Labradors being the most at risk. German shepherds also have a high incidence of hyperplastic nodules and hematomas. However, hemangiosarcoma can affect any breed and size of dog. Small breed dogs with malignant splenic masses are one third as likely to have hemangiosarcoma in comparison with large breed dogs. Clinically and histologically, distinguishing between benign and malignant splenic tumors can be challenging.
Splenic masses are often discovered incidentally or during emergencies induced by hemoabdomen, leading to either urgent surgical intervention or death. However, severe blood loss may complicate the risks associated with surgery. A diagnostic point-of-care test could greatly assist in identifying the type of mass, thereby influencing treatment decisions between surgery and unfortunately often euthanasia. Early detection of splenic masses through routine diagnostic imaging and possibly newly developed blood screening tests could not only prevent emergencies but also enable timely treatment for malignant cases, potentially improving survival rates.
In response of this need, a collection of clinical tissue and serum samples from dogs with splenic masses has been initiated to promote research to develop additional diagnostic tools in veterinary oncology for better management of splenic tumors in dogs. The cohort includes both neutered and non-neutered male and female dogs, aged from 4 years 6 months to 16 years 1 month. Biosamples have been collected according to standard operating procedures (SOPs) in line with ISO standards 20166 and 20184 to ensure that they are suitable for molecular analysis.
Profile of the Spleen diseases in dogs Cohort:
Splenic diseases in dogs
Fixed and native samples from tumor/pathological spleen tissues, (FFPE, OCT, RNAlater, pure) and serum
- Overall: 2021 samples from 99 patients
- Collection ongoing (start 2016)
- untreated
- age: 4 y 6m to 16y 1m
- gender (female, male, neutered)
Clinical data (history, treatment: medication, surgical intervention, laboratory results, imaging data,..), sample management data, images from HE-stains;
Approved by the patient owner
Internal academic research, external academic research, industrial research – cooperations preferred
Tissue samples: CEN/TS 16826 1–3, CEN/TS 16827 1–3
Sample Coordinator: VetBiobank, Ingrid Walter
Email: Ingrid.Walter@vetmeduni.ac.at
Principle investigator: Brigitte Degasperi
Email: Brigitte.Degasperi@vetmeduni.ac.at
Lighthouse collection: Prostate cohort
An exceptional sample collection of the Department of Urology at Biobank Innsbruck (Med Uni Innsbruck)
The “Prostate Cohort” is a sample collection at Biobank Innsbruck (Department of Urology, Med Uni Innsbruck). It is a collection of matched frozen tissue, FFPE tissue and blood samples of malignant and partly benign cases with associated data on histologies, certain laboratory parameters and therapies of prostate cancer specimens over the past 30 years.
Profile of the Prostate Cohort:
Prostate, prostate cancer, prostate benign, PSA
Tissue, serum, urine
From 12,000 patients:
- 435,000 PSA values
- 8,000 tissue samples (frozen/FFPE) (-80°C/RT)
- 180,000 serum samples (-80°C)
- 1,500 urine samples (-80°C)
- 4,600 radical prostatectomy (RPE) surgery data
- 13,000 biopsy data
Male
First line treatment surgical data with histology data of biopsy and RPE samples, follow-up data of control examinations and relapse-data, second line treatment data such as radiation, drug treatment, chemotherapy, etc. and post-treatment data
Declaration of consent available
Yes – in the context of a scientific cooperation
Certified laboratory according to ISO 9001:2015
Principle investigator: Department of Urology Innsbruck
- Prof. Helmut Klocker (retired)
- Dr. Martin Puhr (martin.puhr@i-med.ac.at)
52 projects
- 23 of them are finished/closed/done
- 29 of them are active or waiting to be started
On average 35 publications per year.

The CoVVac Study: COVID-19 vaccination cohort
An outstanding sample collection at Biobank Graz (Med Uni Graz) based on a prospective sample cohort for current research on immune response to COVID-19 vaccines in immunocompromised and healthy individuals.
Study design
This study will characterize and analyze the anit-SARS-CoV-2 humoral and cellular immune response before and after vaccination with a focus on the anti-spike protein response in immunocompromised patients compared to healthy controls. Participants are invited to five visits where samples are collected and one telephone interview:
- 1st visit (60-0 days before vaccination)
- 2nd “visit” is a telephone interview to get information on vaccine reactions.
- 3rd visit (+21-28 days after 2nd vaccination)
- 4th visit (+6 months after 2nd vaccination)
- 5th visit (+3-6 weeks after visit 4 or 3rd vaccination)
- 6th visit (+6 months after visit 4)
Profile of The CoVVac Study:
COVID-19
COVID-19 vaccination, immunocompromised, t-cell immunity, t-cell aging, antibodies
- Serum and saliva (stored at -80 °C)
- PBMC (stored in the gas phase of liquid nitrogen)
Samples will be collected from 189 immunocompromised and 187 healthy controls (6 visits planned)
1371 serum aliquots from 348 participants (May 2021)
- Healthy
- Primary or secondary immunodeficiencies (M05, D38, M34, C90, C91.1, C92, D46)
Serology, immune status, T-cell immunity, nutritive assessment, body fat composition, BMI, date and type of COVID-19 vaccinations, vaccine reactions, medical history, current medication, smoking habits
12/2021
Quality management: ISO 9001:2015 (SOPs)
Principle Investigator: Assoz. Prof. Priv.-Doz. Dr.med.univ. Martin Stradner (biobank-pm@medunigraz.at)
- E Schulz et al. CD19+IgD+CD27- Naïve B Cells as Predictors of Humoral Response to COVID 19 mRNA Vaccination in Immunocompromised Patients; Front. Immunol., 07 December 2021, DOI: 10.3389/fimmu.2021.803742 PMID: 34950155
- ORF Report (Wissenschaft) “Wie wirkt CoV-Impfung bei Immunschwäche?” (15 Dec 2021)
Lighthouse collection: COVID-19 Convalescent Cohort
An exceptional sample collection at Biobank Graz (Med Uni Graz) which is based on a prospective sample cohort for current research on SARS-CoV-2
Summary
Biobank Graz recruited participants (from Austria) who had recovered from COVID-19 and grouped them into two sub cohorts (see profile table). Volunteers were invited to fill in a questionnaire and donate various types of samples at different time points (see study design and table).
This cohort serves for
- the development and validation of new antibody tests and neutralization assays
- the investigation of antibody titres over time
- a better characterization of the course of COVID-19
- the identification of diagnostic or prognostic biomarkers
Study design
Participants are invited five times, i.e. 1st visit (time point 0), 2nd visit (after 1 month), 3rd visit (after 2 months), 4th visit (after 5 months), 5th visit (after 12 months), where the samples are collected. At the 1st visit, donors also fill in a questionnaire concerning symptoms, comorbidities, pre-history and lifestyle.
Profile of the COVID-19 Convalescent Cohort:
COVID-19
COVID-19 recovered patients, SARS-CoV-2, PCR test, antibody test
Serum, EDTA buffy coat, EDTA plasma, lithium heparin plasma, sodium citrate plasma, saliva and nasopharyngeal swabs (stored at -80 °C)
23.739 aliquots from 364 participants as of March 10th
Volunteers who recovered from COVID-19
Sub cohort I (342 participants):
- Persons who were officially tested positive for an infection by PCR test with the SARS-CoV-2 virus, showed symptoms of COVID-19 and recovered.
Sub cohort II (22 participants):
- Persons who lived together or got in contact with a person officially tested positive by PCR test for SARS-CoV-2 and showed symptoms of COVID-19 disease within 14 days, but were not tested themselves, and recovered.
- Persons who showed leading COVID-19 symptoms (fever, dry cough, loss of smell or taste, diarrhoea, fatigue) since December 2019, but were not tested themselves, independent of whether they had contact to a person positive tested for SARS-CoV-2, and recovered.
- Data concerning symptoms, comorbidities, prehistory and lifestyle are collected via questionnaire
- PCR tests and antibody tests are performed
As of now
- Quality management: ISO 9001:2015 (SOPs)
- CEN/TS 16945:2016 (metabolomics in urine, venous blood serum and plasma)
Principle Investigator: Univ.-Prof. Dr. med. univ. Robert Krause (biobank-pm@medunigraz.at)
- Kral S, Banfi C, Niedrist T, Sareban N, Guelly C, Kriegl L, Schiffmann S, Zurl C, Herrmann M, Steinmetz I, Schlenke P, Berghold A, Krause R. Long-lasting immune response to a mild course of PCR-confirmed SARS-CoV-2 infection: A cohort study. J Infect. 2021 Nov;83(5):607-635. doi: 10.1016/j.jinf.2021.08.030. PMID: 34433071.
- Niedrist T, Drexler C, Torreiter PP, Matejka J, Strahlhofer-Augsten M, Kral S, Riegler S, Gülly C, Zurl C, Kriegl L, Krause R, Berghold A, Steinmetz I, Schlenke P, Herrmann M. Longitudinal comparison of automated SARS-CoV-2 serology assays in assessing virus neutralization capacity in COVID-19 convalescent sera. Arch Pathol Lab Med. 2022 Jan 27. doi: 10.5858/arpa.2021-0604-SA. PMID: 35085385.
- Eva M. Matzhold, Günther F. Körmöczi, Chiara Banfi, Marlies Schönbacher, Camilla Drexler, Helmberg, Ivo Steinmetz, Andrea Berghold, Peter Schlenke, Gabriel E. Wagner,
Anja Stoisser, Barbara Kleinhappl, Wolfgang R. Mayr and Thomas Wagner. Lower Levels of ABO Anti-A and Anti-B of IgM, IgG and IgA Isotypes in the Serum but Not the Saliva of COVID-19 Convalescents. J. Clin. Med. 2022, 11(15), 4513, doi: 10.3390/jcm11154513. PMID: 35956128.
Lighthouse collection: Feline Lymphoma Cohort
An exceptional sample collection at VetBiobank Vienna (University of Veterinary Medicine, Vienna)
Summary
Lymphoma is the most common tumor in cats. This neoplasia can develop in every tissue; however, the gastrointestinal tract is in contrast to dogs and people the most frequent localization in the feline species.
The prognosis for cats suffering from intermediate or high-grade lymphoma is still poor and research in this important veterinary area continues to be under-resourced.
The clinical cohort comprises neutered and unneutered male and female patients between 7 months and 15 years. Biospecimens are collected according SOPs. Tissue samples are collected in line with the ISO standards 20166 and 20184 and are therefore suitable for molecular investigations.
Profile of the feline lymphoma cohort:
Lymphoma in cats
Fixed and native samples from tumor and healthy reference tissues (FFPE, glutaraldehyde for electron microscopy, OCT, RNAlater, pure), serum, plasma, urine, feces
Complete set of samples and data with follow up examination for 36 patients available; collection start February 2016 – collection ongoing
untreated, treated
Clinical data (anamnesis, treatment: medication, surgical intervention, laboratory results, imaging data..), sample management data, images from HE stains
Approved by the patient owner
Internal academic research, external academic research, industrial research – cooperation prerred
Tissue samples were collected according to the sample pre-analytics standards: CEN/TS 16826 1-2, CEN/TS 16827 1-3
- Sample Coordinator: VetBiobank, Ingrid Walter (ingrid.walter@vetmeduni.ac.at)
- Principle investigator: Birgitt Wolfesberger (birgitt.wolfesberger@vetmeduni.ac.at)
Lighthouse collection: Colorectal Cancer Whole Slide Image – Survival Cohort“
An exceptional collection by Diagnostic & Research Institute of Pathology at Biobank Graz (both Med Uni Graz)
Summary
The „Colorectal Cancer Cohort (CRC-Cohort)“ is a huge collection of colorectal cancer resection samples from the Institute of Pathology located at Biobank Graz at Medical University of Graz. It consists of various CRC sub-cohorts from colorectal cancer tissue with associated H&E, histological and immunhistochemical stainings where samples have been analysed and supplemented by digital images of tissue slides in various projects.
A very well sub-cohort is the „Colorectal cancer whole slide image cohort“ (CRC WSI cohort) which consists of 3 parts, i.e.:
1. „CRC whole slide image – survival cohort“
2. „CRC whole slide image – vascular invasion cohort“
3. „CRC whole slide image – clinical annotation cohort“
Profile of (1) "CRC whole slide image - survival cohort": This cohort was/is part of several industrial research collaborations e.g. with Google and Roche Diagnostics
Colorectal cancer / ICD-10: C18.0
Colorectal cancer, survival prediction, artificial Intelligence, etc.
- H&E stained FFPE tissue slides (RT storage)
- Immunohistochemistry (IHC) on FFPE tissue slides (RT storage)
- Whole slide images of H&E and IHC FFPE tissue slides
Over 180.000 images (resolution 0.25µm/pixel, over 800 TByte) from 100 000 slides (H&E and IHC staining of FFPE tissue sections) from over 6 400 cases comprising tissue from colorectal cancer resections (tumour and non-tumour colon tissue, resection margins, fatty tissue, and associated lymph nodes)
Inclusion criteria:
- Patients with colorectal cancer as a primary diagnosis (C18), samples from 1984 to 2013
- Patient age: 18 – 100 years
- Tumour stages: II to III
- Ratio female : male = 45:55
- Pathology diagnosis
- TNM staging
- ICD-10 and ICD-O codes
- Survival data
- Meta data from whole slide images
Depending on year of collection with or without informed consent
Access for collaborative research projects depending on ethics committee approval of Medical University of Graz
ISO 9001:2015 (Biobank Graz)
- Wulczyn E, Steiner DF, Moran M, Plass M, Reihs R, Tan F, Flament-Auvigne I, Brown T, Regitnig P, Chen PC, Hegde N, Sadhwani A, MacDonald R, Ayalew B, Corrado GS, Peng LH, Tse D, Müller H, Xu Z, Liu Y, Stumpe MC, Zatloukal K: Interpretable survival prediction for colorectal cancer using deep learning. Mermel CH. NPJ Digit Med. 2021 Apr 19;4(1):71. DOI: 10.1038/s41746-021-00427-2, PMID: 33875798.
- L’Imperio V, Wulczyn E, Plass M et al.: Pathologist Validation of a Machine Learning-Derived Feature for Colon Cancer Risk Stratification. JAMA Netw Open. 2023;6(3):e2254891. DOI:10.1001/jamanetworkopen.2022.54891, PMID: 36917112
- Media article about research collaboration of Med Uni Graz and Google on revolutionary findings with relevance for diagnostics: algorithms are declaring war on cancer (Kleine Zeitung 2021)
Industry collaboration
Univ.-Prof. Dr. med. univ. Kurt Zatloukal (kurt.zatloukal@medunigraz.at)
Lighthouse collection: Colorectal Cancer Whole Slide Image – Vascular Invasion Cohort
An exceptional collection by Diagnostic & Research Institute of Pathology at Biobank Graz (both Med Uni Graz)
Summary
The „Colorectal Cancer Cohort (CRC-Cohort)“ is a huge collection of colorectal cancer resection samples from the Institute of Pathology located at Biobank Graz at Medical University of Graz. It consists of various CRC sub-cohorts from colorectal cancer tissue with associated H&E, histological and immunhistochemical stainings where samples have been analysed and supplemented by digital images of tissue slides in various projects.
A very well sub-cohort is the „Colorectal cancer whole slide image cohort“ (CRC WSI cohort) which consists of 3 parts, i.e.:
1. „CRC whole slide image – survival cohort“
2. „CRC whole slide image – vascular invasion cohort“
3. „CRC whole slide image – clinical annotation cohort“
Profile of (2) "CRC whole slide image - vascular invasion cohort": Sub-collection of the CRC collection that was part of the EU H2020 project ADOPT BBMRI-ERIC (grant agreement number: 676550)
Colorectal cancer / ICD-10: C18.0
Colorectal cancer, survival prediction, artificial Intelligence, etc.
- H&E stained FFPE tissue slides (RT storage)
- (Immuno)histochemistry (IHC) on FFPE tissue slides, Elastica van Giesson staining, vascular endothelial marker IHC (CD-31) (RT storage)
- Whole slide images of H&E and IHC FFPE tissue slides
Approx. 800 whole slide images (resolution 0.25µm/pixel) from ~ 800 slides (H&E, histochemical and IHC staining of FFPE tissue sections) from over 260 cases comprising colorectal tumour tissue
Inclusion criteria:
- Patients with colorectal cancer as a primary diagnosis (C18), samples from 1984 to 2013
- Patient age: 18 – 100 years
- Tumour stages: I to IV
- Female, male
- Pathology diagnosis
- TNM staging
- ICD-10 and ICD-O codes
- Clinical data
- Survival data
- Meta data from whole slide images
Depending on year of collection with or without informed consent
Access for collaborative research projects depending on ethics committee approval of Medical University of Graz
ISO 9001:2015 (Biobank Graz)
Univ.-Prof. Dr. med. univ. Kurt Zatloukal (kurt.zatloukal@medunigraz.at)
Sub-collection of the CRC collection that was part of the EU H2020 project ADOPT BBMRI-ERIC (grant agreement number 676550)
Lighthouse collection: Colorectal Cancer Whole Slide Image – Clinical Annotation Cohort
An exceptional collection by Diagnostic & Research Institute of Pathology at Biobank Graz (both Med Uni Graz)
Summary
The „Colorectal Cancer Cohort (CRC-Cohort)“ is a huge collection of colorectal cancer resection samples from the Institute of Pathology located at Biobank Graz at Medical University of Graz. It consists of various CRC sub-cohorts from colorectal cancer tissue with associated H&E, histological and immunhistochemical stainings where samples have been analysed and supplemented by digital images of tissue slides in various projects.
A very well sub-cohort is the „Colorectal cancer whole slide image cohort“ (CRC WSI cohort) which consists of 3 parts, i.e.:
1. „CRC whole slide image – survival cohort“
2. „CRC whole slide image – vascular invasion cohort“
3. „CRC whole slide image – clinical annotation cohort“
Profile of (3) "CRC whole slide image - clinical annotation cohort": Sub-collection of the CRC collection that was part of the EU H2020 project ADOPT BBMRI-ERIC (grant agreement number: 676550)
Colorectal cancer / ICD-10: C18.0
Colorectal cancer, survival prediction, artificial Intelligence, etc.
- H&E stained FFPE tissue slides (RT storage)
- Immunohistochemistry (IHC) on FFPE tissue slides (RT storage)
- Whole slide images of H&E and IHC FFPE tissue slides
Approx. 1800 whole slide images (resolution 0.25µm/pixel) from ~800 slides (H&E and IHC staining of FFPE tissue sections) from over 100 cases comprising tissue from colorectal cancer resections (tumour and non-tumour colon tissue, resection margins, fatty tissue, and associated lymph nodes)
Inclusion criteria:
- Patients with colorectal cancer as a primary diagnosis (C18), samples from 1984 to 2013
- Age: 18 – 100 years
- Stages: II and III
- Female, male
- Pathology diagnosis
- TNM staging
- ICD-10 and ICD-O codes
- Clinical data
- Survival data
- Meta data from whole slide images
Depending on year of collection with or without informed consent
Access for collaborative research projects depending on ethics committee approval of Medical University of Graz
ISO 9001:2015 (Biobank Graz)
Univ.-Prof. Dr. med. univ. Kurt Zatloukal (kurt.zatloukal@medunigraz.at)
Sub-collection of the CRC collection that was part of the EU H2020 project ADOPT BBMRI-ERIC (grant agreement number 676550)
*Data associated with ADOPT CRC Cohort:
- Sex
- Participation in clinical study (yes/no)
- Age at primary diagnosis
- Time of recurrence (metastasis)
- Family history of cancer
- Other diseases: other cancers, Inflammatory bowel disease, intestinal polyps, other disease requiring therapy (e.g., cardio-vascular, endocrine. musculoskeletal)
- Tumor markers (e.g., CEA) (if available)
- Vital status and survival information: timestamp of last update of vital status, overall survival status
- Surgery: time difference between initial diagnosis and surgery, surgery radicality, type of surgery
- Pharmacotherapy (REQUIRED if occurred): start of pharmacotherapy (relative date referring to the primary diagnosis), scheme of pharmacotherapy
- Targeted therapy (REQUIRED if occurred): start of targeted therapy (relative date referring to the primary diagnosis), end of targeted therapy (relative date referring to the primary diagnosis)
- Radiation therapy (REQUIRED if occurred): start of radiotherapy (relative date referring to the primary diagnosis), end of radiotherapy (relative date referring to the primary diagnosis)
- Response to therapy: specific response; the response is linked to the patient and specified by a timestamp. This is to avoid need to specify to which therapy the response is, since there might be combination of different therapies
- Molecular markers (if available): Microsatellite instability, Mismatch repair gene expression – IHC array for different genes (common for 3; if applicable), risk situation (only HNPCC), RAS mutation status (if applicable), BRAF, PIC3CA. HER2 mutation status (optional)
- Histopathology: pTNM, UICC staging, WHO grading, histological classification (according to WHO), localization of the tumor, high resolution digital images (resolution < 0.25 Microns/pixel) of the tumor, resection margin and lymph nodes
- Diagnostic exam: result of colonoscopy, array of diagnostic methods (results of liver imaging, lung imaging, MRI, CT; if occurred)
LEAD Cohort (Austrian LEAD Study)
An outstanding collection at BBMRI.at partner MedUni Wien Biobank
Summary
The Biobank of the Medical University of Vienna harbours the sample cohort of the “Austrian LEAD-Study” collected at the Ludwig Bolzmann Institute. It is a population based cohort and one of the largest long-term studies in Austria on the topic of lung health.
The Lung, hEart, sociAl, boDy (LEAD) Study is a longitudinal, observational, epidemiological cohort study aiming to investigate respiratory health through life. The survey includes a representative sample of the Austrian general population between 6 and 80 years of age and focuses on three areas:
- Normal and pathologic lung growth and development (natural history of lung function).
- Genetic, environmental and socioeconomic risk factors for abnormal lung function.
- Extrapulmonary manifestations of abnormal lung function and related comorbidities (cardiovascular disease, metabolic disorders, depression and anxiety).

Lighthouse collection: BioPersMed Cohort
An outstanding interdisciplinary sample collection at Biobank Graz
Summary
“BioPersMed” stands for “Biomarkers for Personalised Medicine in Common Metabolic Disorders.
This prospectively collected cohort comprises a huge number of samples and data being collected and used since 2010 (still ongoing). Aim is to detect and define specific characteristics and personalized biomarkers of patients at risk for cardiovascular and metabolic diseases and to follow them for future developments.
The “BioPersMed Cohort” includes the research areas endocrinology & metabolism and cardiology. Diabetes, fatty liver disease, osteoporosis or cardiovascular diseases are only some of the topics in the focus of this cohort.
The samples meet the requirements of the sample quality standards that were published by the European Committee for Standardization (CEN).
Profile of the BioPersMed Cohort:
Diabetes, fatty liver disease, osteoporosis, cardiovascular diseases
Biomarker, endocrinology, metabolism, cardiology
Plasma (EDTA, Lithium Heparin, Sodium Citrate), serum, buffy coat; urine, 24h urine of healthy and diseased patients
- 1,017 probands (in 4 follow-up visits): healthy and diseased
- 355,635 aliquots (as per August 2024)
collection ongoing
- Healthy and diseased
- Age: adults 35+ years
- Gender: 50/50 female/male
Clinical records/diagnosis, smoking and lifestyle anamnesis, follow-up laboratory results, follow-up clinical data
Cooperation only
- Sample quality: CEN/TS 16945:2016 – Molecular in vitro diagnostic examinations. Specifications for pre-examination processes for metabolomics in urine, venous blood serum and plasma
- Quality management: ISO 9001:2015 (SOPs)
- Haudum CW, Kolesnik E, Colantonio C, Mursic I, Url-Michitsch M, Tomaschitz A, Glantschnig T, Hutz B, Lind A, Schweighofer N, Reiter C, Ablasser K, Wallner M, Tripolt NJ, Pieske-Kraigher E, Madl T, Springer A, Seidel G, Wedrich A, Zirlik A, Krahn T, Stauber R, Pieske B, Pieber TR, Verheyen N, Obermayer-Pietsch B, Schmidt A. Cohort profile: ‘Biomarkers of Personalised Medicine’ (BioPersMed): a single-centre prospective observational cohort study in Graz/Austria to evaluate novel biomarkers in cardiovascular and metabolic diseases. BMJ Open. 2022 Apr 7;12(4):e058890. doi: 10.1136/bmjopen-2021-058890. PMID: 35393327; PMCID: PMC8991072.
- Reintar S, Pöchhacker M, Obermayer A, Eberhard K, Zirlik A, Verheyen N, von Lewinski D, Scherr D, Hutz B, Haudum CW, Pieber TR, Sourij H, Obermayer-Pietsch B. Urinary C-Peptide to Creatinine Ratio (UCPCR) as Indicator for Metabolic Risk in Apparently Healthy Adults-A BioPersMed Cohort Study. Nutrients. 2023 Apr 25;15(9):2073. doi: 10.3390/nu15092073. PMID: 37432211; PMCID: PMC10181310.
- Tandl V, Haudum C, Eberhard K, Hutz B, Foessl I, Kolesnik E, Zirlik A, von Lewinski D, Scherr D, Verheyen N, Pieber T, Obermayer-Pietsch B. AMH in Males: Effects of Body Size and Composition on Serum AMH Levels. J Clin Med. 2023 Jul 4;12(13):4478. doi: 10.3390/jcm12134478. PMID: 37445513; PMCID: PMC10342968.
- Scientific director: Barbara Obermayer-Pietsch (barbara.obermayer@medunigraz.at)
- Partners: Thomas Pieber, Albrecht Schmidt (biobank-pm@medunigraz.at)

PoCOsteo Osteoporosis Cohort
An outstanding osteoporosis sample collection at Biobank Graz (Med Uni Graz)
Summary
“PoCOsteo” stands for Point-of-care in-office device for identifying individuals at high risk of Osteoporosis and osteoporotic fracture.
It is being collected (starting May 2018) in the context of the HORIZON 2020 project PoCOsteo (Proposal number 767325) which aims at developing point-of-care in-vitro devices to measure and/or quantify proteomic and genomic factors in human whole blood.
The clinical cohort includes male and female patients fifty years and above and ranges from healthy (per definition) to those who experienced multiple fragility fractures due to secondary osteoporosis.
It comprises untreated patients as well as those treated for osteoporosis, with or without concomitant diseases such as hypertension, diabetes etc.
The samples meet the requirements of the sample quality standards “CEN/TC 140 Molecular in vitro diagnostic examinations – Specifications for preexamination processes (CEN/TS) for metabolomics in urine, venous blood serum and plasma” (CEN/TS 16945:2016) that were published by the European Committee for Standardization (CEN).
Profile of the PoCOsteo Cohort:
Endocrinology, osteoporosis, osteoporotic fractures
Biomarker development & detection
Serum, EDTA-plasma and buffy coat (stored at -80°C)
1,000 probands
Collection start in May 2018 – collection ongoing
- Healthy and diseased, treated and untreated for osteoporosis with or without concomitant diseases (e.g. hypertension, diabetes, …)
- Age: adults > 50 years
- Gender: female and male
From the following time-points:
- study inclusion
- after 12 months
- possibly after 24 months
Bone mineral density (BMD) of the hip and the lumbar spine as well as vertebral fracture assessment (VFA) by Dual X-ray absorptiometry (DXA), grip strength (hydraulic hand dynamometer), and a routine blood test including renal and hepatic parameters together with a lipid profile
Cooperation preferred
- Sample quality: CEN/TS 16945:2016 – Molecular in vitro diagnostic examinations. Specifications for pre-examination processes for metabolomics in urine, venous blood serum and plasma
- Quality management: ISO 9001:2015 (SOPs)
Principle Investigator: Hans Dimai, Medical University of Graz, Department of Internal Medicine, Division of Endocrinology and Metabolism (biobank-pm@medunigraz.at, hans.dimai@medunigraz.at)

PERFORM Cohort
An outstanding sample collection at Biobank Graz (Med Uni Graz)
Summary
“PERFORM” stands for Personalised Risk assessment in Febrile illness to Optimise Real-life Management across the European Union. It represents a pediatric cohort of samples from children and is collected in the context of the PERFORM EU Horizon 2020 project.
The collection aims to improve the diagnostics in febrile children and adolescents and to develop a comprehensive management plan for febrile patients in Europe. It helps to improve the diagnostics in febrile children and adolescents.
The “PERFORM cohort” comprises PAXgene stabilized and EDTA blood samples (whole blood, plasma, pellet), cerebrospinal fluid, throat swab, urine and stool. It includes children below the age of 18 (both genders) with i) fever >38ºC, or a history of fever (within 3 days), or ii) suspected of infection.
Profile of PERFORM Cohort:
Febrile illness
ETDA blood (plasma, pellet and whole blood), blood in PAXgene tubes, cerebrospinal fluid, throat swab, urine, and stool stored at -80°C
Samples from 500 patients
Children <18 years
- with fever >38ºC, or a history of fever (within 3 days), in whom the attending clinician determines the need for blood sampling or whom parents give consent for bloods taken for research purposes
- suspected of infection (included: full spectrum of disease severity, and with co-morbidities)
Specific study IC
Cooperation only
Quality management: ISO 9001:2015 (SOPs)
ORF Report “Lebensbedrohlich krank: Neuer Schnelltest”
Principle Investigator: Univ. Prof. Dr. Werner Zenz, Pediatric and Adolescent University Hospital, Department of General Pediatrics Medical University of Graz (perform@medunigraz.at, biobank-pm@medunigraz.at)

INTERFAST Cohort
An outstanding sample collection at Biobank Graz (Med Uni Graz)
Summary
“INTERFAST” stands for Intermittent Fasting (i.e. a dietary regimen of alternating fasting and “feeding” cycles, e.g. practised by alternate day fasting). The INTERFAST collection represents cohort of blood and urine samples from healthy donors who have undergone different types of intermittent fasting and is collected in the context of the INTERFAST project (www.interfast.at).
The cohort supports studies that aim to investigate the effects of repeating fasting periods on human physiology, aging process and molecular cellular processes in humans.
This includes both studying
- long term effects of fasting (as donors are included in the cohort, who already practice alternate day fasting (ADF) for a defined time period) as well as
- short term effects of this nutritional intervention (i.e. intermittent fasting)
The INTERFAST cohort comprises serum, plasma and urine samples.
Profile of the INTERFAST Cohort:
Intermittent fasting, alternate day fasting (ADF), effects on autophagy, human physiology, aging process and molecular-cellular processes
Serum, plasma and urine samples stored at -80°C
90 donors:
- 30 subjects cohort – 2 study visits
- 60 subjects RCT – 4 study visits
Cohort (Alternate day fasting for at least 6 months before the start of the trial – 30 subjects) as well as randomized control-trial (60 subjects – randomized to either intervention group (alternate day fasting) or control group with no intervention 1:1).
- Adults 35 – 65 years; ratio female:male = 57:43
Further inclusion criteria: - Body mass index in the range of 22.0 – 30.0 kg/m2
- Fasting blood glucose <110mg/dL (without medication)
- LDL-cholesterol <180 mg/dL (without medication)
- Blood pressure <140/90 mmHg (without medication)
- Stable weight (change <± 10%) for 3 months immediately
Exclusion criteria: see INTERFAST cohort details
- Demographics
- Medical history
- Concomitant medication
- Vital signs
- Physical examination
- Blood sampling with oral glucose tolerance test (oGTT)
- Electrocardiogram (ECG)
Cooperation preferred
Quality management: ISO 9001:2015 (SOPs)
Principle Investigator: Assoz. Prof. Dr. Harald Sourij (biobank-pm@medunigraz.at)
“Intermittent Fasting (Alternate Day Fasting) in Healthy, Non-obese Adults: Protocol for a Cohort Trial with an Embedded Randomized Controlled Pilot Trial” (Advances in Therapy, August 2018, Volume 35, Issue 8) PMID: 30046988.

Paracelsus 10,000 Cohort
An outstanding population-based collection at BBMRI.at partner PMU / SALK
Summary
Paracelsus 10,000 Cohort is a population-based collection from 5,000 male and 5,000 female probands from Salzburg (Austria). It comprises numerous different types of samples and data from diverse analyses and different follow up visits.
Purpose of this cohort is to serve the following study aims:
- Collection of valid epidemiological data on the state of health of the Salzburg population
- Clarification of the interaction of genetic disposition and lifestyle factors in the development of degenerative diseases
- Development of well-directed prevention processes for the population of Salzburg
- Strengthening of the research location Salzburg and Austria
Profile of the Paracelsus 10,000 Cohort:
Plasma (EDTA, citrate and heparin), serum, EDTA blood for DNA isolation, buffy Coat, urine, stool samples
Representative sample of 10 000 participants (5.000 male, 5.000 female) by 03/2020
- 40-49 years: 2700 participants
- 50-59 years: 4300 participants
- 60-69 years: 3000 participants
From the city of Salzburg and surrounding townships
Male-female ratio 1:1
1. Basic investigations of all participants:
- Questionnaires on diet, exercise, socio-economic anamnesis, depression, cognitive performance, quality of life, stress and environmental factors
- Anthropometry: height, weight, abdominal girth
- Resting blood pressure, heart rate, resting 12-channel ECG
- Laboratory parameters: Lipid profile, Apo-B, Apo-AI, Lp(a), FBS, HbA1c, Electrolytes, CR, Urea, LFT, Fe-Status, hsCRP, fibrinogen, BC, TSH, PSA (men), Fasting-Insulin
- Urine: chemistry, albumin/creatinine ratio
- Ultrasound of the carotides
2. Additional investigations for the intensively phenotyped subgroup (50 to 59 years):
- Laboratory parameters: fasting proinsulin, free fatty acids, adiponectin, vitamin D
- Determination of insulin sensitivity and beta cell function by frequently sampled OGTT (with simultaneous determination of insulin, C-peptide and blood glucose at the time points 0 min., 30 min., 60 min., 90 min. and 120 min.)
- Pulmonary function test
- Pulse wave analysis
- Multi-frequency body composition B.I.A.
- Measurement of intima-media thickness and plaque score of carotid arteries
- Measurement of the coronary artery calcium score
- 24h blood pressure measurement
- 7-day nutrition protocol
- 7-day movement monitoring using movement sensors
- Ankle-brachial index
- Hand grip
- 6 meter walking test
3. Additional investigations for two participants per day (50 to 59 years):
- Coronary Ca-score
- Body fat distribution and bone density (measured with DEXA-scan)
- Ergospirometry
- Ultrasound of the liver
- Prof. Dr. Bernhard Paulweber (b.paulweber@salk.at)
- Dr. Ludmilla Kedenko PhD (l.kedenko@salk.at)
Vanessa Natalie Frey, Patrick Langthaler, Emanuel Raphaelis et al. Paracelsus 10,000: A Prospective Cohort Study Based On The Population of Salzburg, Austria. Rationale, Objectives And Study Design, 17 August 2021, PREPRINT (Version 1). doi: 10.21203/rs.3.rs-740574/v1.