News

25.06.2025
Legal
Legal Q&A: What a researcher is allowed to do with biobanked human samples?
BBMRI.at Legal Helpdesk answers a question

18.06.2025
Events
| News
BBMRI.at QM Workshop 2025: Quality through collaboration
BBMRI.at advances biobank quality through its third national QM Workshop

13.06.2025
EDU & Training
| Events
| Legal
| News
| Project
| Quality
Biobanking e-course and serious game for non-biobankers – presented by SCIBIOEU partner Med Uni Graz (BBMRI.at)
Free-to-use science outreach tools to inform about biobanking
06.06.2025
Cohorts
| For Researchers
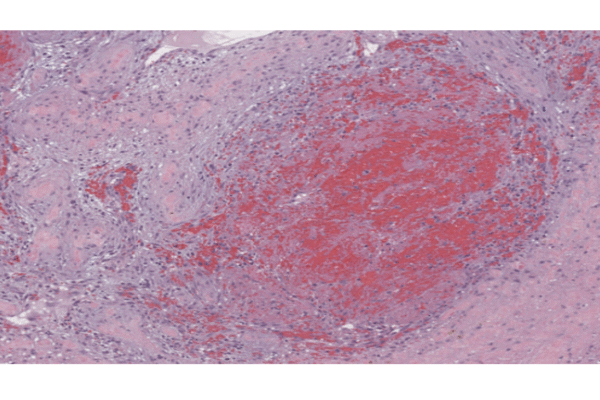
Spleen diseases in dogs
An exceptional sample collection at VetBiobank Vienna (University of Veterinary Medicine, Vienna)

22.05.2025
Events
| News
From one health, green biobanking, big data and AI to data security
BBMRI.at’s contributions to the EBW25

21.05.2025
Legal
Legal Q&A: Biobank donor rights, data safety, and data regulations
BBMRI.at Legal Helpdesk answers a question

20.05.2025
News
Now available: SPIDIA4P Newsletter – 2024/2025
News about ISO/CEN standards, sample pre-analytics & proficiency testing

13.05.2025
Legal
Legal Q&A: Key questions about informed consent (IC) in biobanking
BBMRI.at Legal Helpdesk answers a question

08.05.2025
Legal
Legal Q&A: Informed consent for pet owners in veterinary biobanking
BBMRI.at Legal Helpdesk answers a question

25.04.2025
For Researchers
| News
Call for Transnational Cancer Research Services – Apply Now
canSERV Open Call for researchers in and outside the EU

11.04.2025
News
“BoB – Best of Biotech” is back – submit your business ideas
11th edition of the biotech business plan competition

11.04.2025
Legal
Legal Q&A: What is the status of broad consent in Austria?
BBMRI.at Legal Helpdesk answers a question

25.06.2025
Legal
Legal Q&A: What a researcher is allowed to do with biobanked human samples?
BBMRI.at Legal Helpdesk answers a question

18.06.2025
Events
| News
BBMRI.at QM Workshop 2025: Quality through collaboration
BBMRI.at advances biobank quality through its third national QM Workshop

13.06.2025
EDU & Training
| Events
| Legal
| News
| Project
| Quality
Biobanking e-course and serious game for non-biobankers – presented by SCIBIOEU partner Med Uni Graz (BBMRI.at)
Free-to-use science outreach tools to inform about biobanking

06.06.2025
Cohorts
| For Researchers
Spleen diseases in dogs
An exceptional sample collection at VetBiobank Vienna (University of Veterinary Medicine, Vienna)

22.05.2025
Events
| News
From one health, green biobanking, big data and AI to data security
BBMRI.at’s contributions to the EBW25
Events

