BBMRI.at Legal Knowledge Base
Legal Q&A: Is there a Biobank Act within the EU?
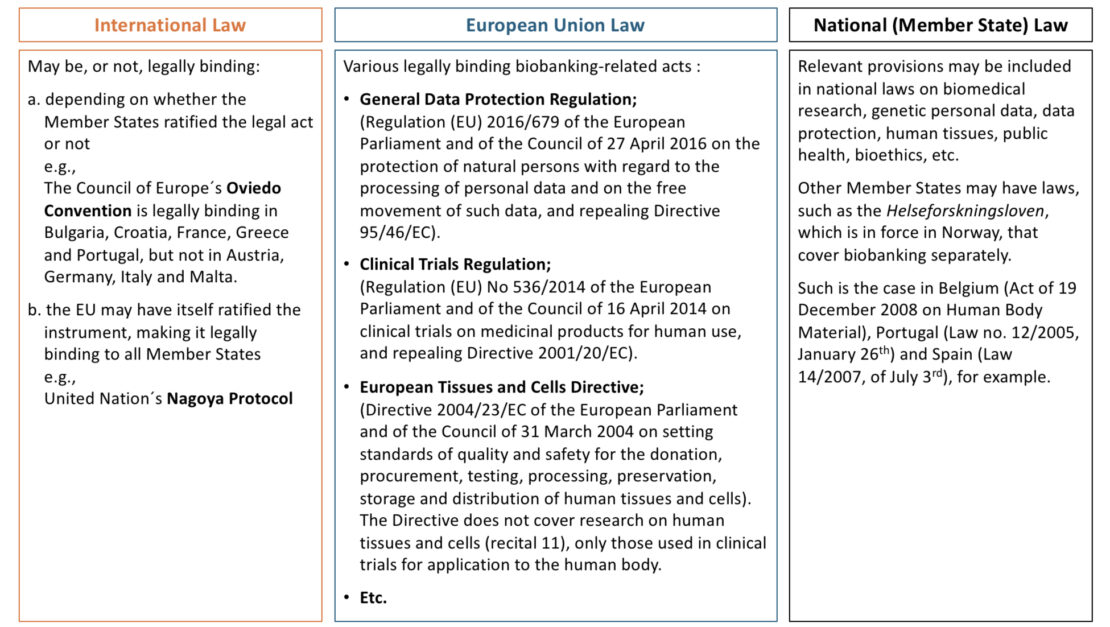
Currently, there is no single EU-wide regulation that explicitly regulates biobanks and biobanking. There is, however, an existing EU framework in addition to national regulations of EU Member States. This framework’s status quo is illustrated here by the BBMRI.at Legal Helpdesk.
BBMRI.at Legal Helpdesk Service answers
The BBMRI.at Legal Helpdesk Service – operated by legal experts from BBMRI.at partner UNIVIE- answers questions on legal and regulatory matters around biobanking and/or using biological samples and data. This service is offered to BBMRI.at partners to support them, as biobanking and research using biological samples and data (e.g. human, animal/veterinary, microbial, etc.) may raise legal questions. Answers provided by UNIVIE to legal questions are published in the BBMRI.at Knowledge Base.
QUESTION:
Is there a Biobank Act within the EU?
ANSWER:
Currently, there is no single European Union (EU)-wide regulation that explicitly regulates biobanks and biobanking. However, an existing EU framework that applies to some aspects of biobanking – such as a Regulation on personal data protection – which coexists with the EU Member States national regulations on the matter. This framework’s status quo is illustrated as follows.
This fragmented legal framework (1) (shown in the table above) derives partially from the fact that, historically, the subject-matter of biomedical research was legislated on by Member States and not by the EU (2). Perhaps the EU’s most influential legal act on biobanking is the General Data Protection Regulation (3) (GDPR). By regulating data protection – and specifically introducing a normative regime for data protection within scientific research – the GDPR has been influential over scientific research legislation and practice in the EU Member States (4). Nevertheless, the implementation of the GDPR in the different Member States results in distinct regulatory outcomes.
Upcoming EU legislative acts – such as the European Health Data Space (EHDS) Regulation (5) (with one of its main features being secondary use of electronic health data which will be valuable to biobanks), the Artificial Intelligence (AI) Act (6) (which, inter alia, includes provisions governing how secondary use of electronic health data may be employed for AI training and testing) and the “SoHO Regulation” (7) – are also set to impact biobanking within the EU and harmonise some of its segments.
The legislation in force in different EU Member States which specifically addresses biobanks, is presented in the table below.
EU State
Member Legislative Act
kutatások, valamint a biobankok működésének szabályairól
amended by Lei n.º 26/2016, de 22 de Agosto)
The legislation of other Member State‘s (such as Austria, France, Germany, and Poland) addresses biobanking through a combination of various acts covering issues such as biomedical research, data protection, bioethics, public health, etc (8).
The inexistence of a Regulation covering biobanking in the EU results in the circumstance of structural issues regarding the biobanking activity being governed differently in the EU Member States. A point of specific concern has been the legal limitations created by each of the Member States regarding international (within and beyond the EU) sample sharing. Some countries prohibit the transfer of samples abroad – which impairs cross-border scientific research – whilst others are more permissive in this regard (9).
As the current heterogeneity may hinder the development of collaborative biomedical research within the EU (and between the EU and third countries)(10) and uniformization can prove to be too much of an ambitious project short term and not feasible in the present circumstances (11) (due to different Member States traditions in approaching the matter of biomedical research and different perceptions in key issues, such as the desirable breadth of consent that should be gathered for research activities, for example), harmonisation through “[…] the development of common guidelines, standard operating procedures (SOPs), and harmonization methodologies […] is crucial” (12). Academic scholars have also pointed out other areas in which harmonisation within the EU would be beneficial: such is the case of the ‘vocabulary’ used within the biobanking community (‘samples’, ‘anonymisation’, etc.) (13), which has been previously addressed at a BBMRI-ERIC level (14) and the classification of the different types of biobanks, which also varies within the EU (15).
The reality of an EU-wide approach to the regulation of the biobanking activity faces various challenges and garners degrees of support which differ widely among the populations of different EU Member States (16). Other European organisations – such as the Council of Europe – have issued guidelines and recommendations aiming to promote the harmonisation of the regulation on biobanking within the scope of the Council of Europe’s Member States and beyond (see §2 of the Preamble of the Recommendation (17)).
Sources:
(1) SLOKENBERGA, Santa. “Setting the Foundations: Individual Rights, Public Interest, Scientific Research and Biobanking.” GDPR and Biobanking: Individual Rights, Public Interest and Research Regulation across Europe (2021), pp. 11-30, p. 12.
(2) Ibidem, p. 13.
(3) Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data, and repealing Directive 95/46/EC (General Data Protection Regulation).
(4) SLOKENBERGA, Santa, Op. Cit., p. 17.
(5) EUROPEAN COMMISSION, Proposal for a Regulation of the European Parliament and of the Council on the European Health Data Space – COM/2022/197 final (Document 52022PC0197).
(6) European Parliament legislative resolution of 13 March 2024 on the proposal for a regulation of the European Parliament and of the Council on laying down harmonised rules on Artificial Intelligence (Artificial Intelligence Act) and amending certain Union Legislative Acts (COM(2021)0206 – C9-0146/2021 – 2021/0106(COD)). The proposal “[…] does not apply to AI systems or AI models, including their output, specifically developed and put into service for the sole purpose of scientific research and development.” (article 2(6)).
(7) EUROPEAN COMMISSION, Proposal for a Regulation of the European Parliament and of the Council on standards of quality and safety for substances of human origin intended for human application and repealing Directives 2002/98/EC and 2004/23/EC (Document 52022PC0338). The Regulation will not apply to research using substances of human origin when that research does not involve human application (recital 60).
(8) TZORTZATOU, Olga, et al. Biobanking across Europe post-GDPR: a deliberately fragmented landscape. Springer International Publishing, 2021, pp. 397-414, p. 404.
(9) BEIER, Katharina & LENK, Christian. “Biobanking strategies and regulative approaches in the EU: recent perspectives.” Journal of Biorepository Science for Applied Medicine, 2015, pp. 69-81, p. 78.
(10) KAYE, Jane. “Do we need a uniform regulatory system for biobanks across Europe?.” European Journal of Human Genetics 14.2, 2006, pp. 245-248, p. 247.
(11) BEIER, Katharina & LENK, Christian, Op. Cit., p. 72.
(12) GOTTWEIS, Herbert. Biobanks for Europe: A challenge for governance: Report of the expert group on dealing with ethical and regulatory challenges of international Biobank Research, European Union, 2012, p. 20.
(13) FRANSSON, Martin N., et al. “Toward a common language for biobanking.” European Journal of Human Genetics. 23.1, 2015, pp. 22-28.
(14) Vide: Lexicon – BBMRI Wiki (wikidot.com)
(15) BEIER, Katharina & LENK, Christian, Op. Cit., p. 70.
(16) BIOBANKING AND BIOMOLECULAR RESOURCES RESEARCH INFRASTRUCTURE (BBMRI), Biobanks and the Public. Governing Biomedical Research Resources in Europe, 2013, p. 44. Available from: BBMRI_Master_Galley.indd (bbmrieric.eu) (access: 09/05/2024).
(17) COUNCIL OF EUROPE, Recommendation CM/Rec(2016)6 of the Committee of Ministers to member States on research on biological materials of human origin.
Disclaimer: this commentary aims to provide a summary of the main ethical and legal issues related to the questions put by interested stakeholders and to direct them to the relevant legal provisions that are applicable. It does not, however, preclude from reading the official sources of legislation relating to the subject matters of this document as well as those quoted by the authors and does not constitute legal advice.