News

22.05.2025
Events
| News
From one health, green biobanking, big data and AI to data security
BBMRI.at’s contributions to the EBW25

21.05.2025
Legal
Legal Q&A: Biobank donor rights, data safety, and data regulations
BBMRI.at Legal Helpdesk answers a question

20.05.2025
News
Now available: SPIDIA4P Newsletter – 2024/2025
News about ISO/CEN standards, sample pre-analytics & proficiency testing

13.05.2025
Legal
Legal Q&A: Key questions about informed consent (IC) in biobanking
BBMRI.at Legal Helpdesk answers a question

08.05.2025
Legal
Legal Q&A: Informed consent for pet owners in veterinary biobanking
BBMRI.at Legal Helpdesk answers a question

25.04.2025
For Researchers
| News
Call for Transnational Cancer Research Services – Apply Now
canSERV Open Call for researchers in and outside the EU

11.04.2025
News
“BoB – Best of Biotech” is back – submit your business ideas
11th edition of the biotech business plan competition

11.04.2025
Legal
Legal Q&A: What is the status of broad consent in Austria?
BBMRI.at Legal Helpdesk answers a question

10.04.2025
For Researchers
| News
BBMRI.at Biobanking eNEWS
News and current affairs regarding biobanking: February – March 2025
10.04.2025
News
| Publication
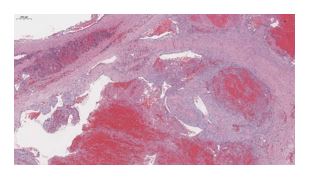
Advances in diagnosis of splenic tumors in dogs using analysis of miRNA from veterinary biobank samples
BBMRI.at publication on differentiating splenic tumors in dogs

08.04.2025
Events
Register now: HELT 2025 – One Health Workshop on legal, environmental, and technological challenges in digital health research
BBMRI.at participation at HELT 2025 Symposium in Brussel, register now

06.04.2025
Events
Register now: AI in biobanking: legal & IT challenges – with BBMRI.at expert presentation
BBMRI-ERIC ELSI DIALOGUES webinar on navigating the future (11 Apr 2025; 11-12 CEST)

22.05.2025
Events
| News
From one health, green biobanking, big data and AI to data security
BBMRI.at’s contributions to the EBW25

21.05.2025
Legal
Legal Q&A: Biobank donor rights, data safety, and data regulations
BBMRI.at Legal Helpdesk answers a question

20.05.2025
News
Now available: SPIDIA4P Newsletter – 2024/2025
News about ISO/CEN standards, sample pre-analytics & proficiency testing

13.05.2025
Legal
Legal Q&A: Key questions about informed consent (IC) in biobanking
BBMRI.at Legal Helpdesk answers a question

08.05.2025
Legal
Legal Q&A: Informed consent for pet owners in veterinary biobanking
BBMRI.at Legal Helpdesk answers a question
Events

