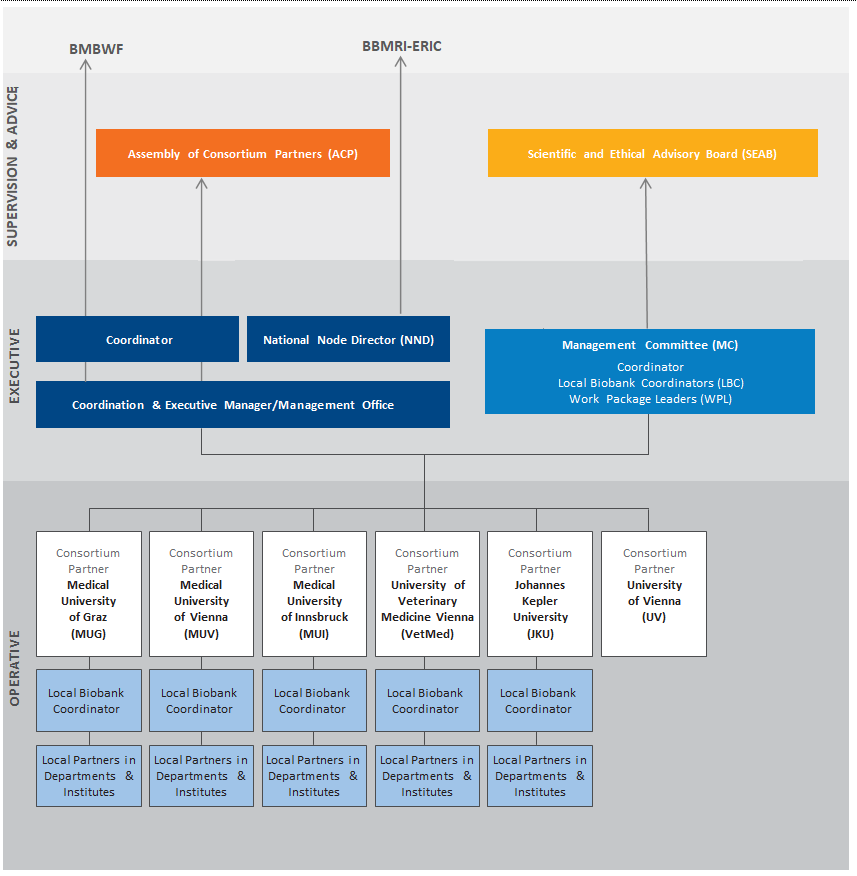
Governance Structure & Organization of BBMRI.at
The governance structure of the Austrian biobanking infrastructure BBMRI.at resembles that of the European BBMRI-ERIC.
Management Committee
The Management Committee consists of the National Node Directors, the Local Biobank Coordinators of each consortium partner and of Work Package Leaders.
BBMRI.at Coordinator: Luka Brcic (Medical University of Graz)
BBMRI.at National Node Director: Georg Göbel (Medical University of Innsbruck)
BBMRI.at National Node Director Deputy: Luka Brcic (Medical University of Graz)
BBMRI.at Executive Manager and Coordinator Deputy: Cornelia Stumptner (Medical University of Graz)
Work Package Leaders
Work Package (WP) Titles
Work Package Leaders (WPLs)
Coordination Office
Operational / Project Manager: Cornelia Stumptner (Medical University of Graz)
Local Biobank Coordinates
Consortium Partner Biobank
Assembly of Consortium Partners (ACP)
The ACP consists of representatives of each BBMRI.at consortium partner
ACP Representative
Consortium Partner
External Scientific and Ethical Advisory Board (SEAB)
The SEAB consists of experts who supervise the work of BBMRI.at and give advice to the Management Committee.